Quality Issues & CAPA Management - K2C Solutions
K2C solution dedicated to Quality Issues and CAPA (Corrective and Preventive Action) is designed to meet the regulation requirements defined by ICH (International Conference of Harmonization) and local regulations. Many observations during FDA or other agency inspections are related to CAPA system, that is very hard to be managed without an appropriate software solution.
K2C CAPA solution facilitates your company compliance, returning at the meantime a lot of benefits in term of process efficiency. Our solution is powerful but simple, avoiding you put any extra effort to manage your CAPA system.
The CAPA module includes the following main features:
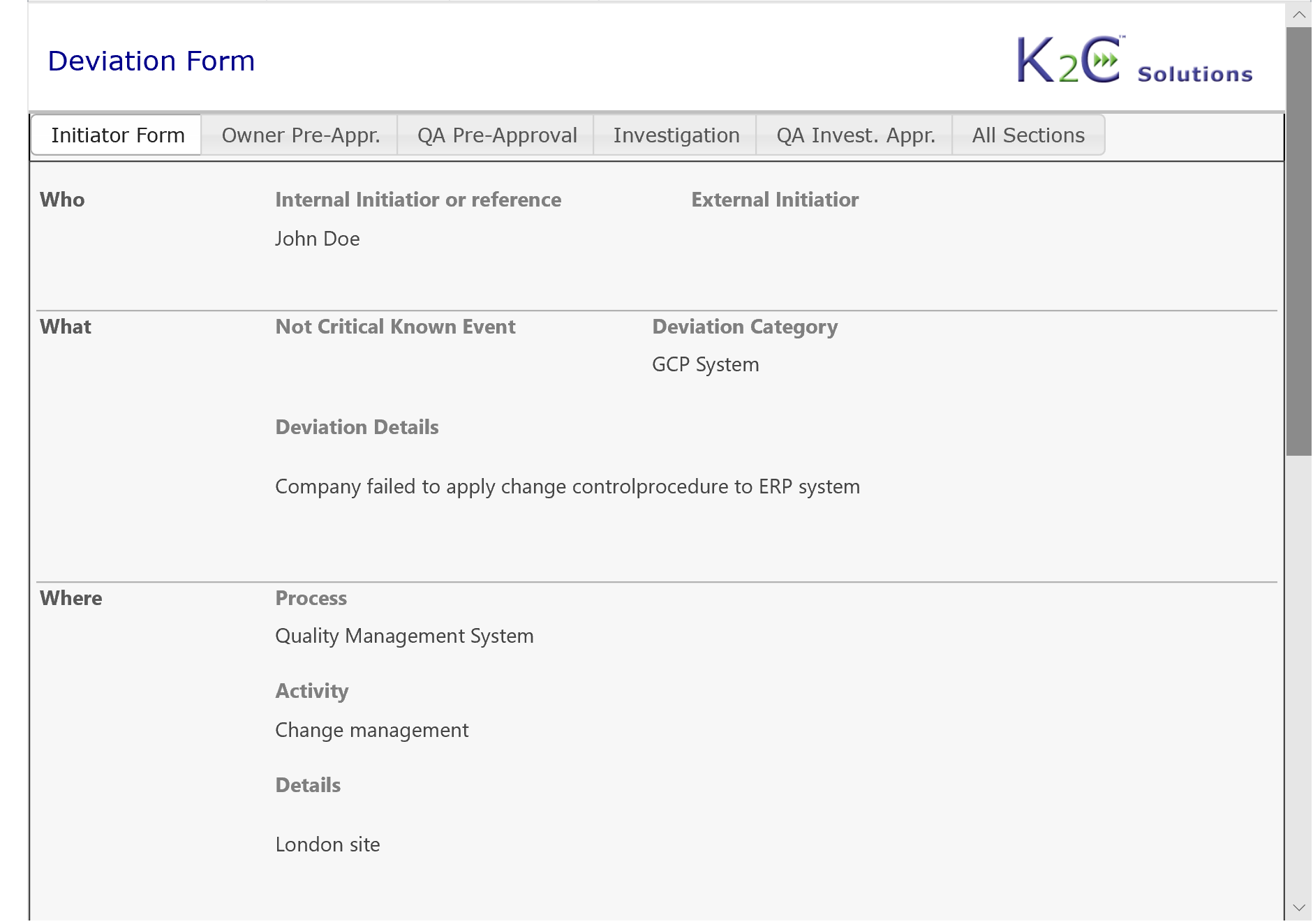
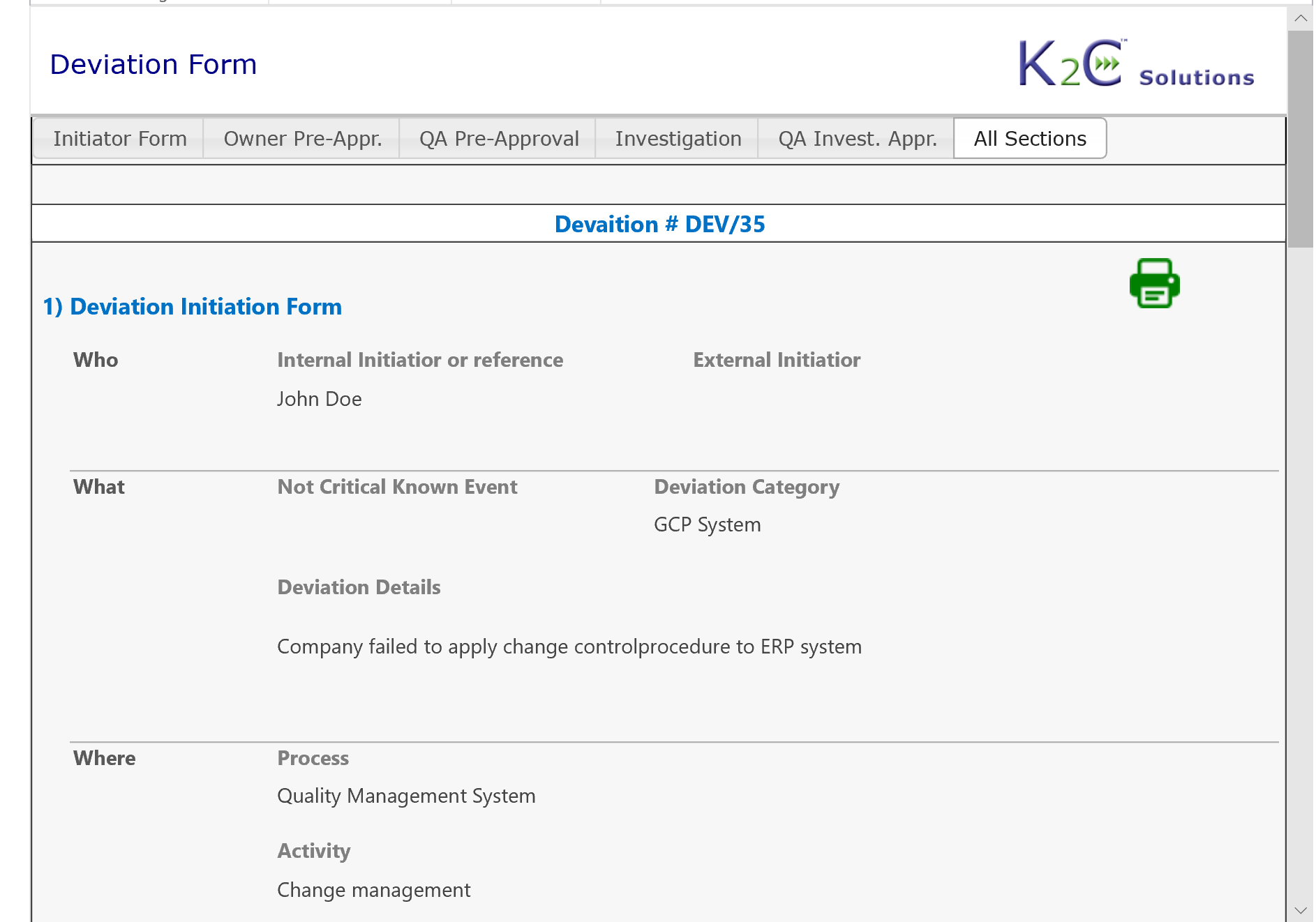
- Identification and communication of a "Quality Issue" (OOS, OOT, Deviation, Complaint etc.)
- Management of documentation associated to the Quality Issue
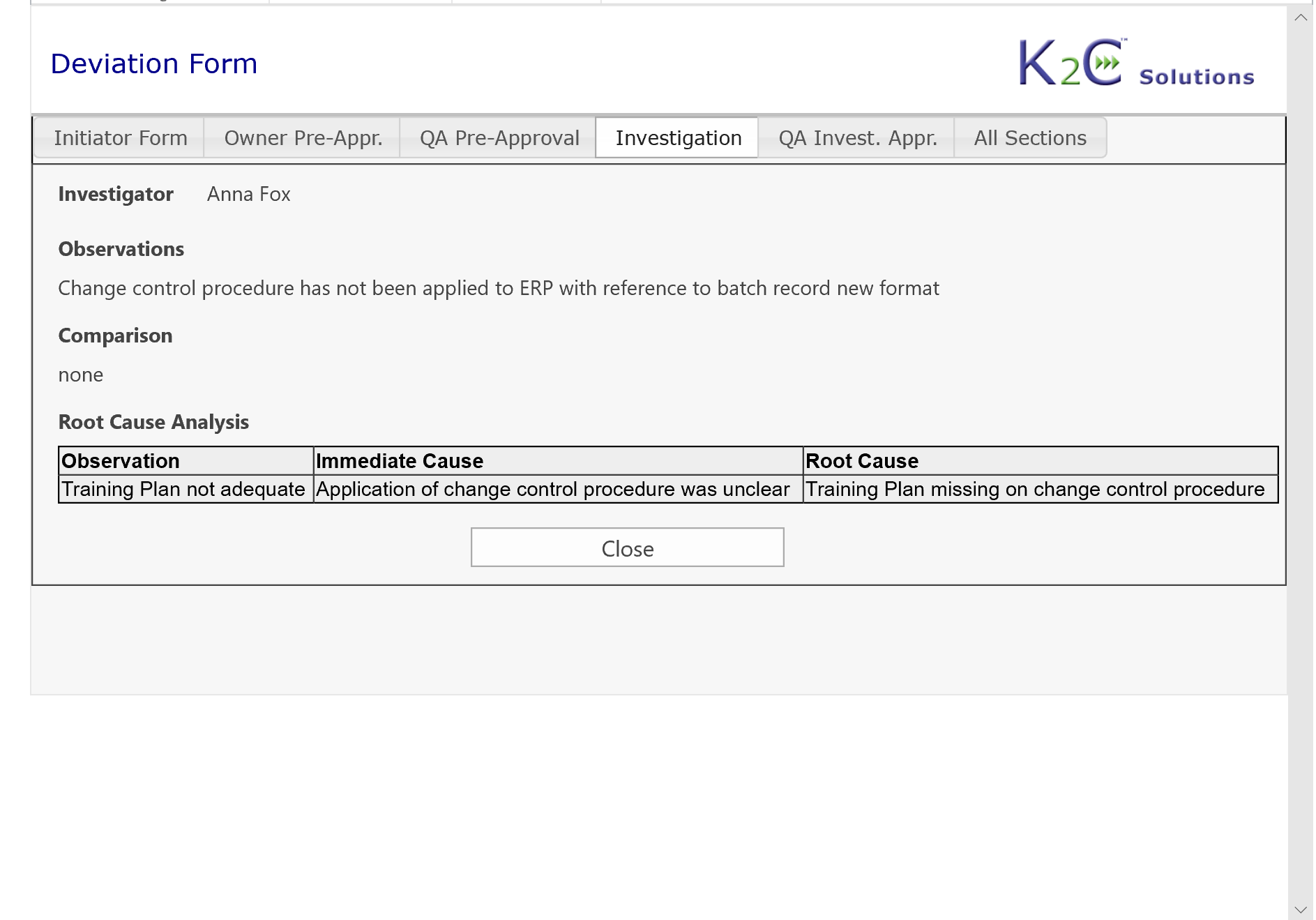
- Root cause analysis
- Management of Investigation and related Report
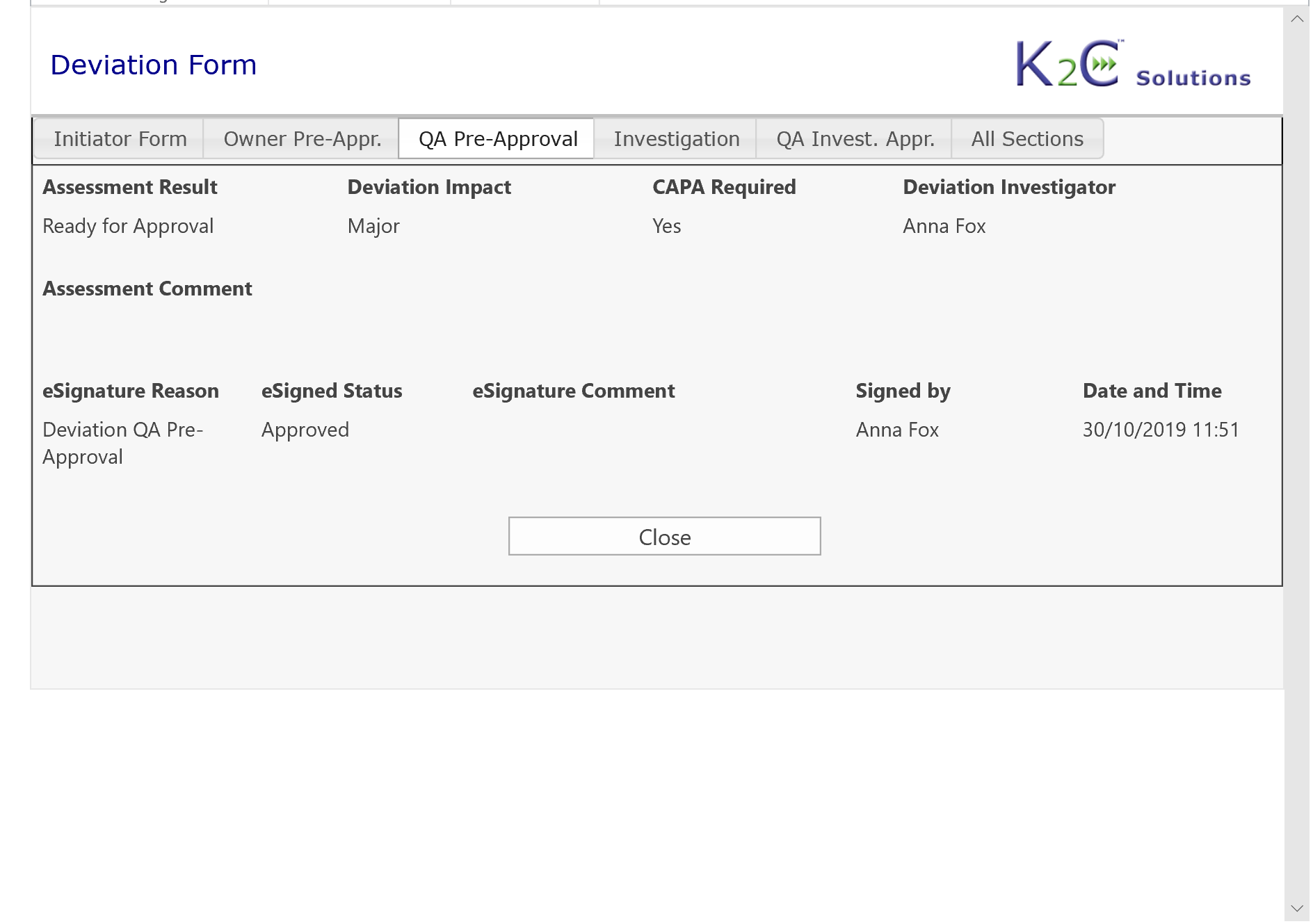
- Risk Assessment
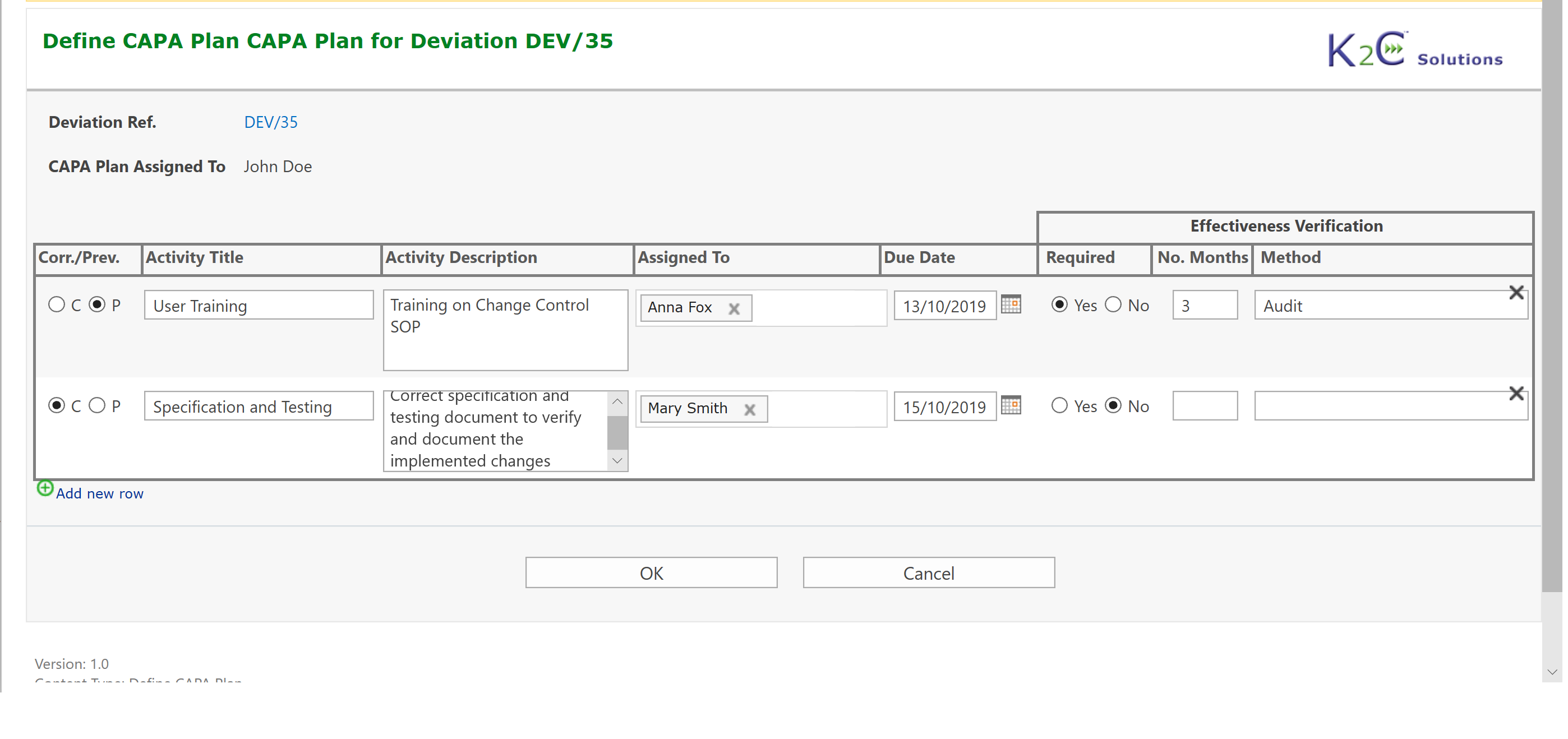
- Definition of CAPA Plan as a set of Preventive/Corrective Actions
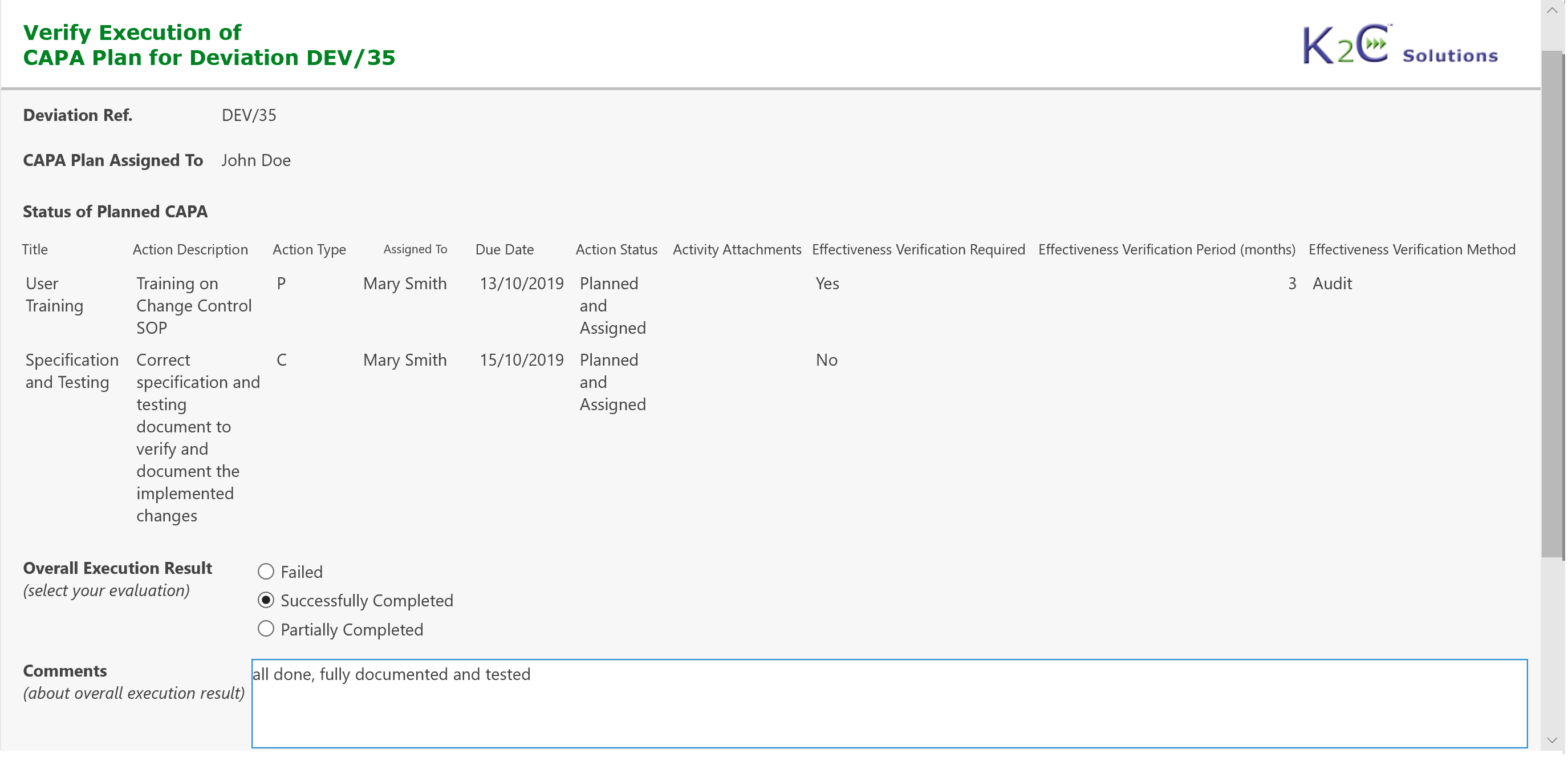
- Monitoring and Follow-up of Preventive/Corrective Actions
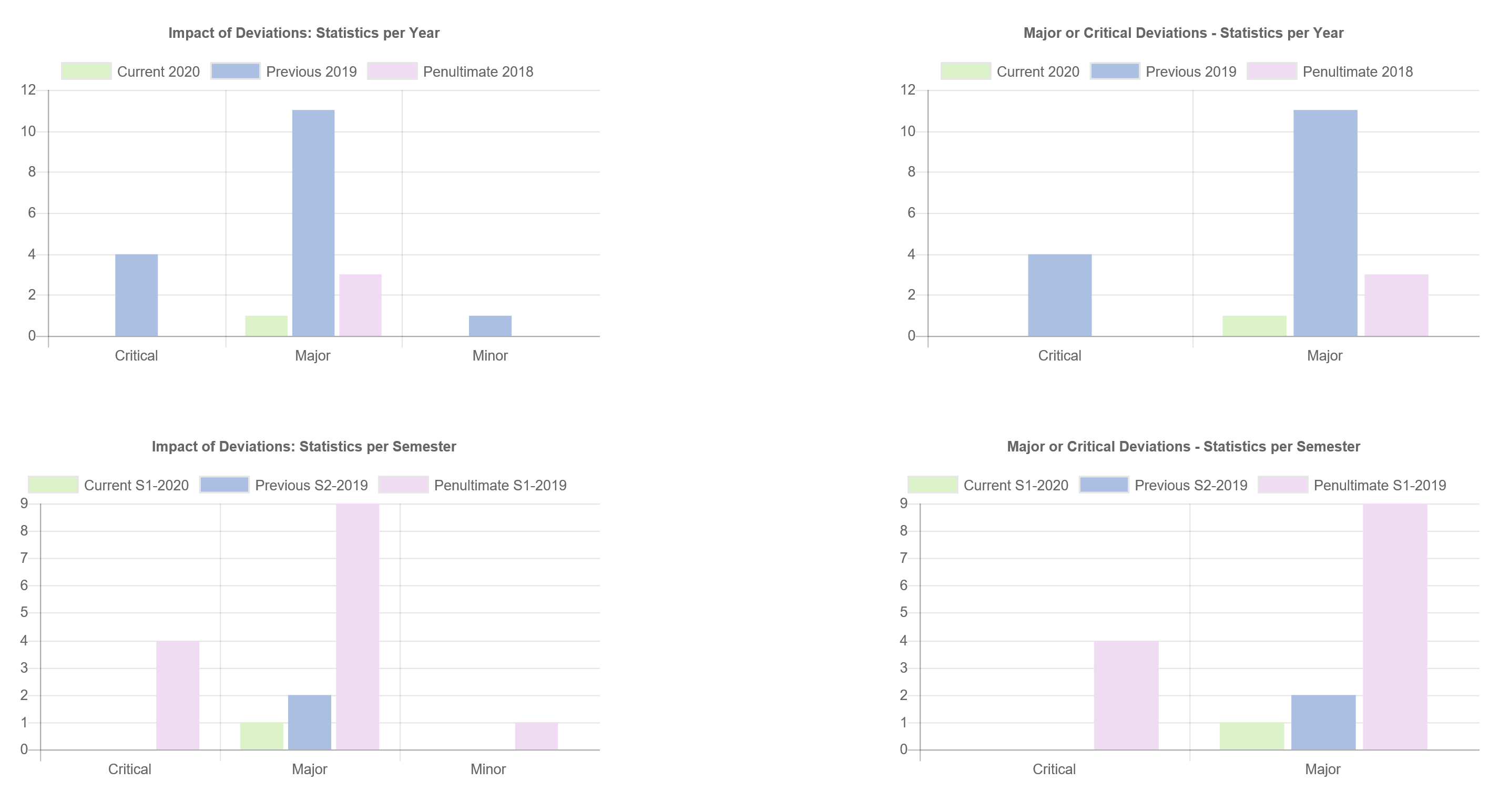
- Dashboards, charts and statistics
- Management of Communication and alerts/reminder
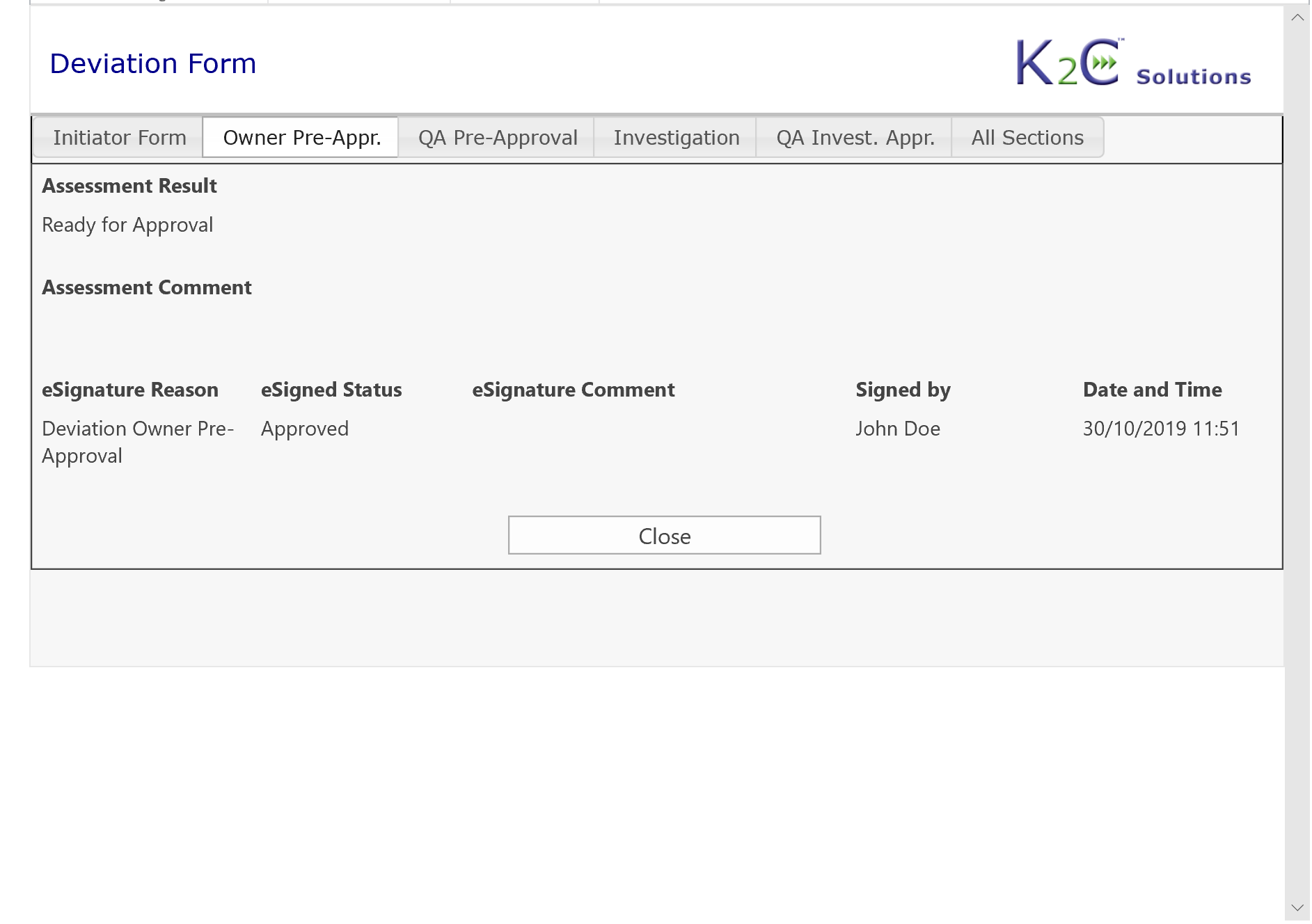
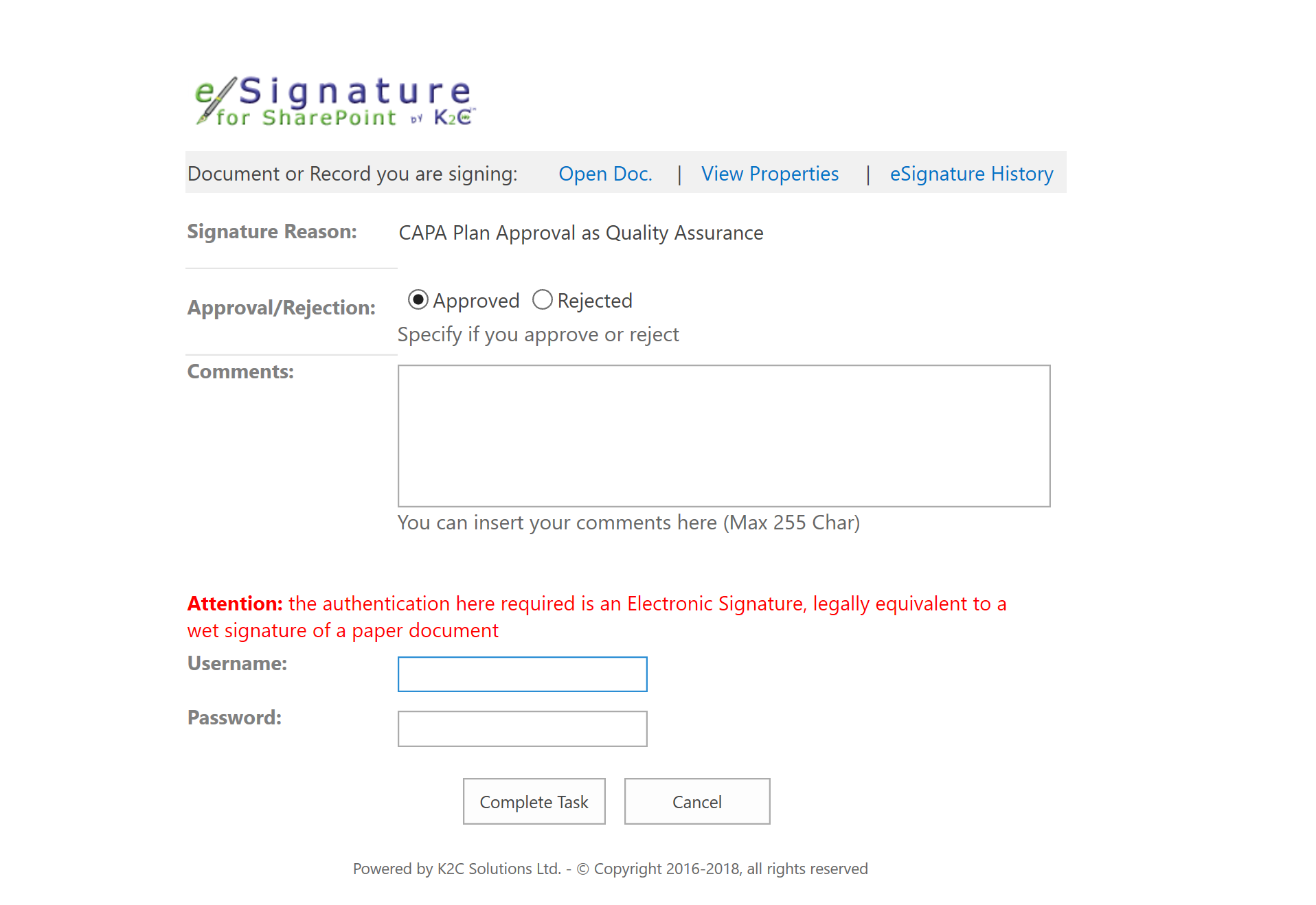
- Approval workflows including Electronic Signature compliant with 21 CFR part 11
K2C Deviation and CAPA Management solution also includes all required features to comply with Electronic Record and Signature regulation (21 CRF part 11).
Contact us for more information or to request for a demo.