K2C Change Management Solution
K2C Change Management Solution is based on SharePoint and allows to manage the change control of systems and processes, strongly reducing time and costs dedicated to these kind of activities, that are usually required by organizations working under a Quality System and specific regulations applicable to their business (GMP, GCP, GLP etc.).
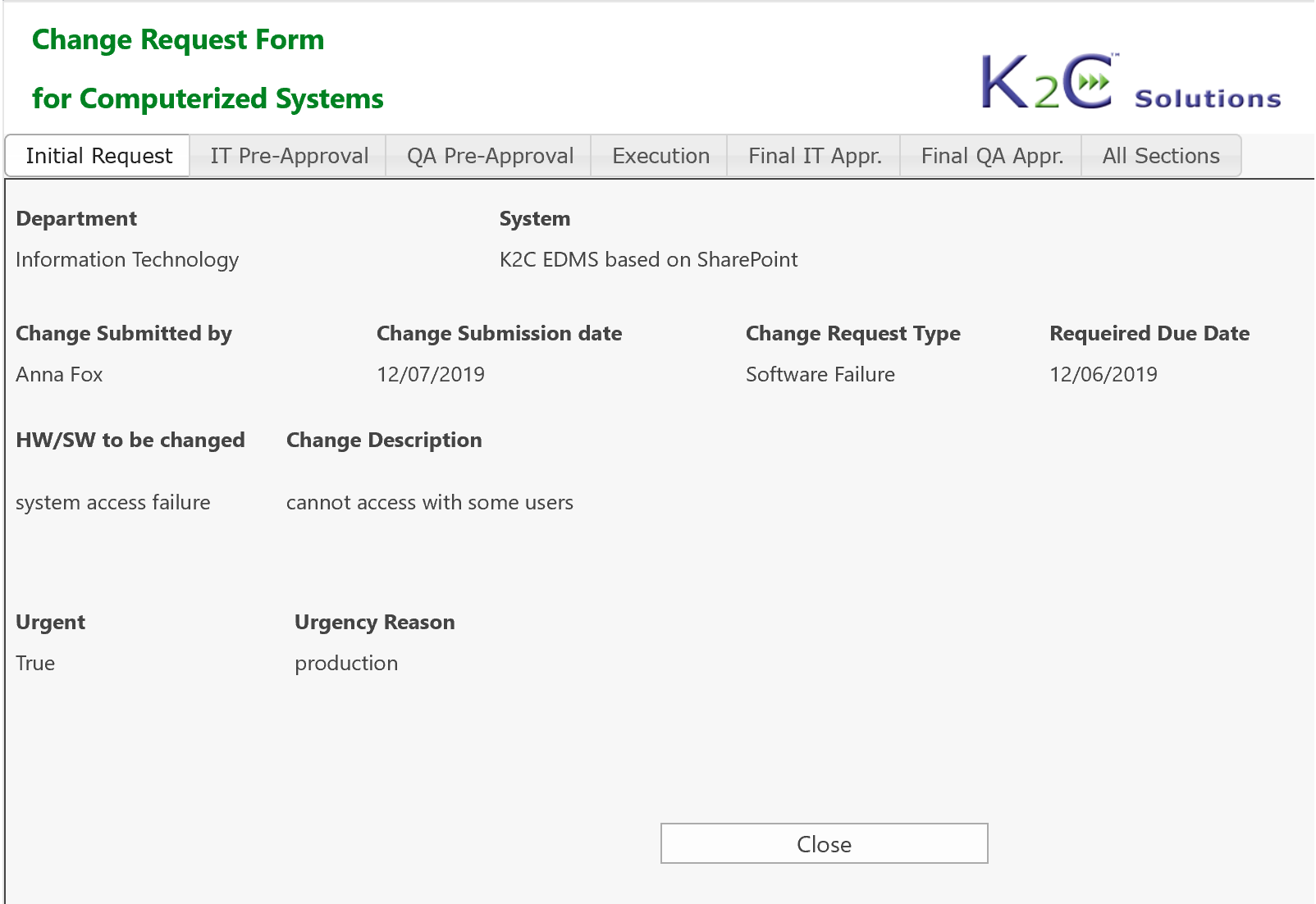
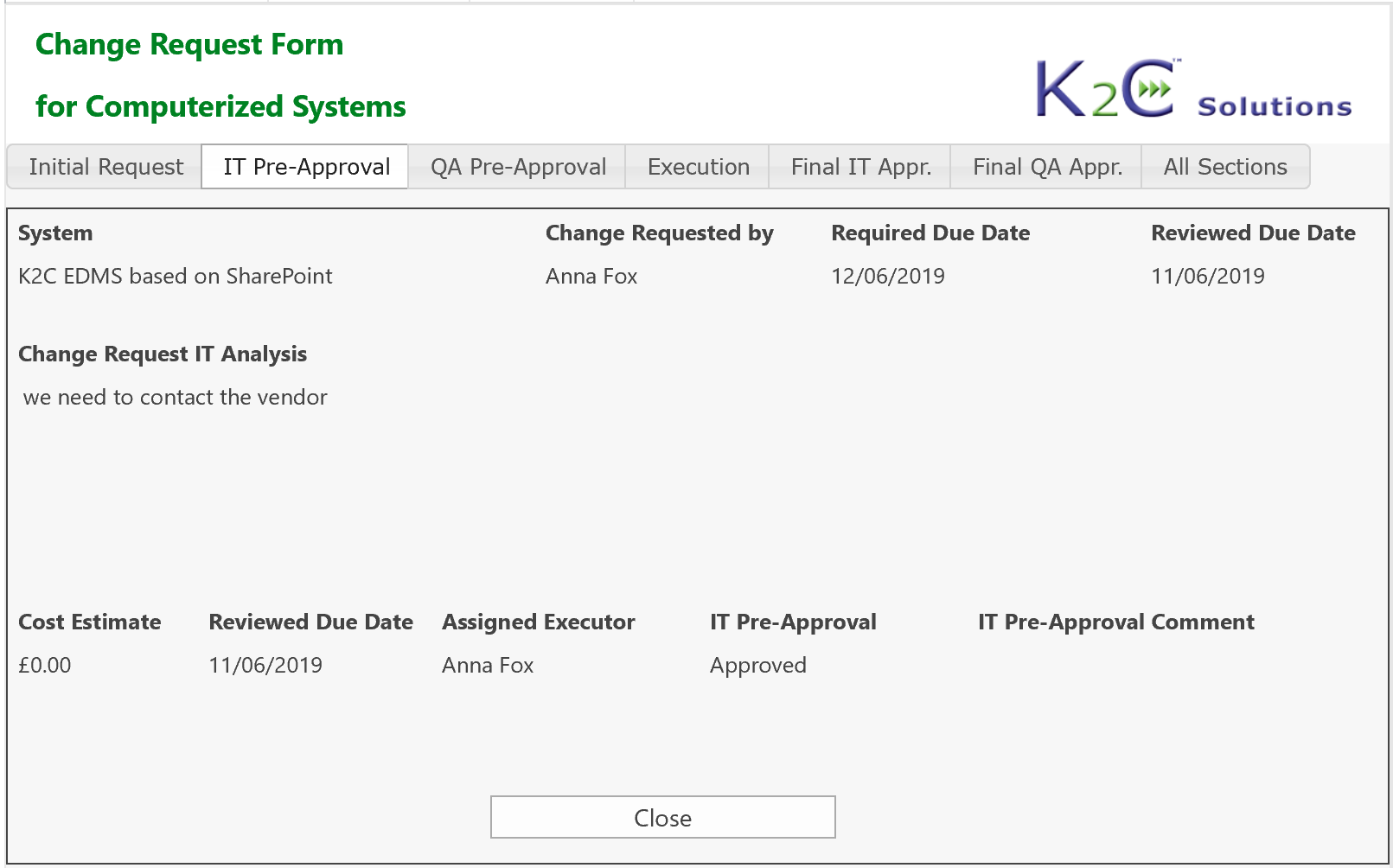
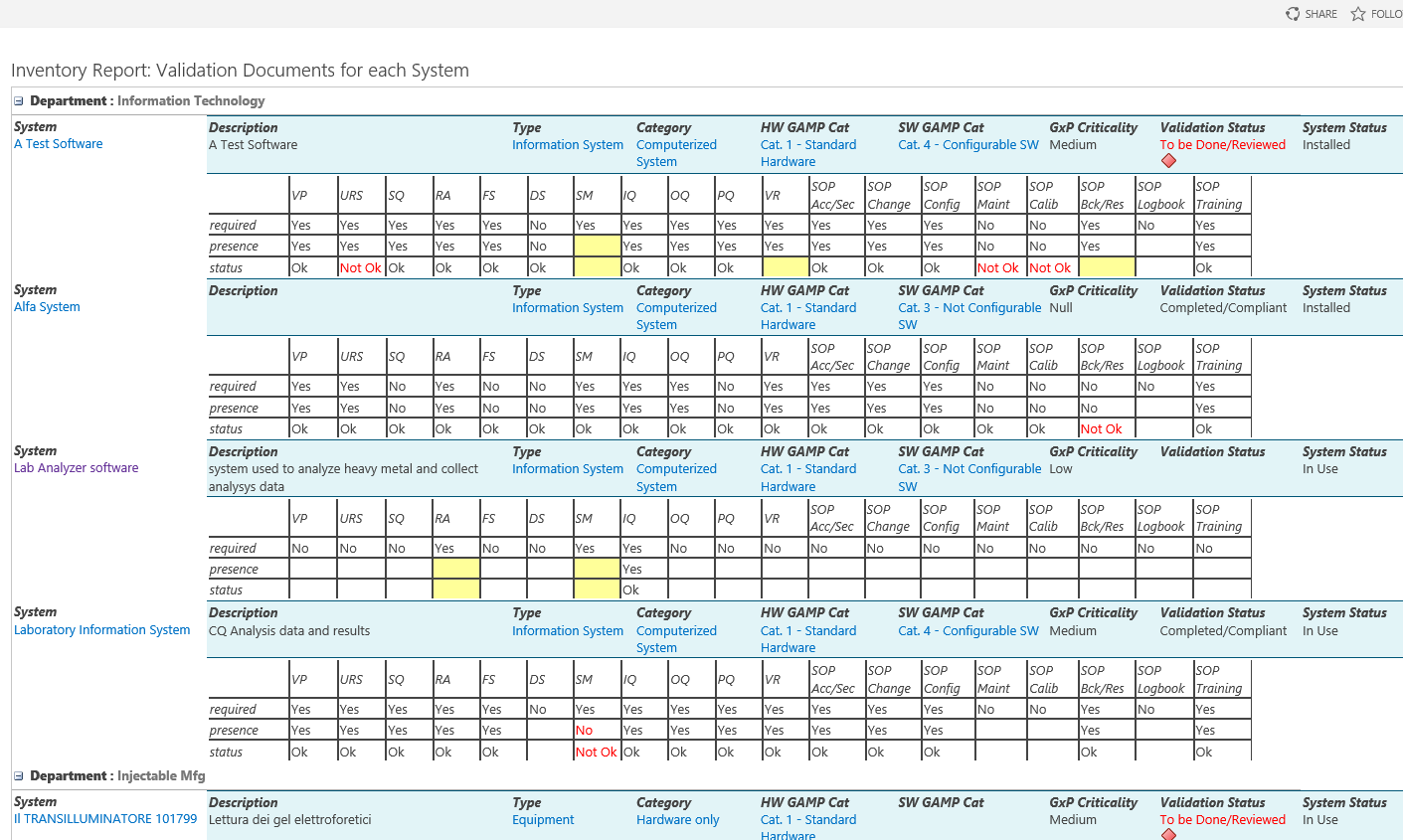
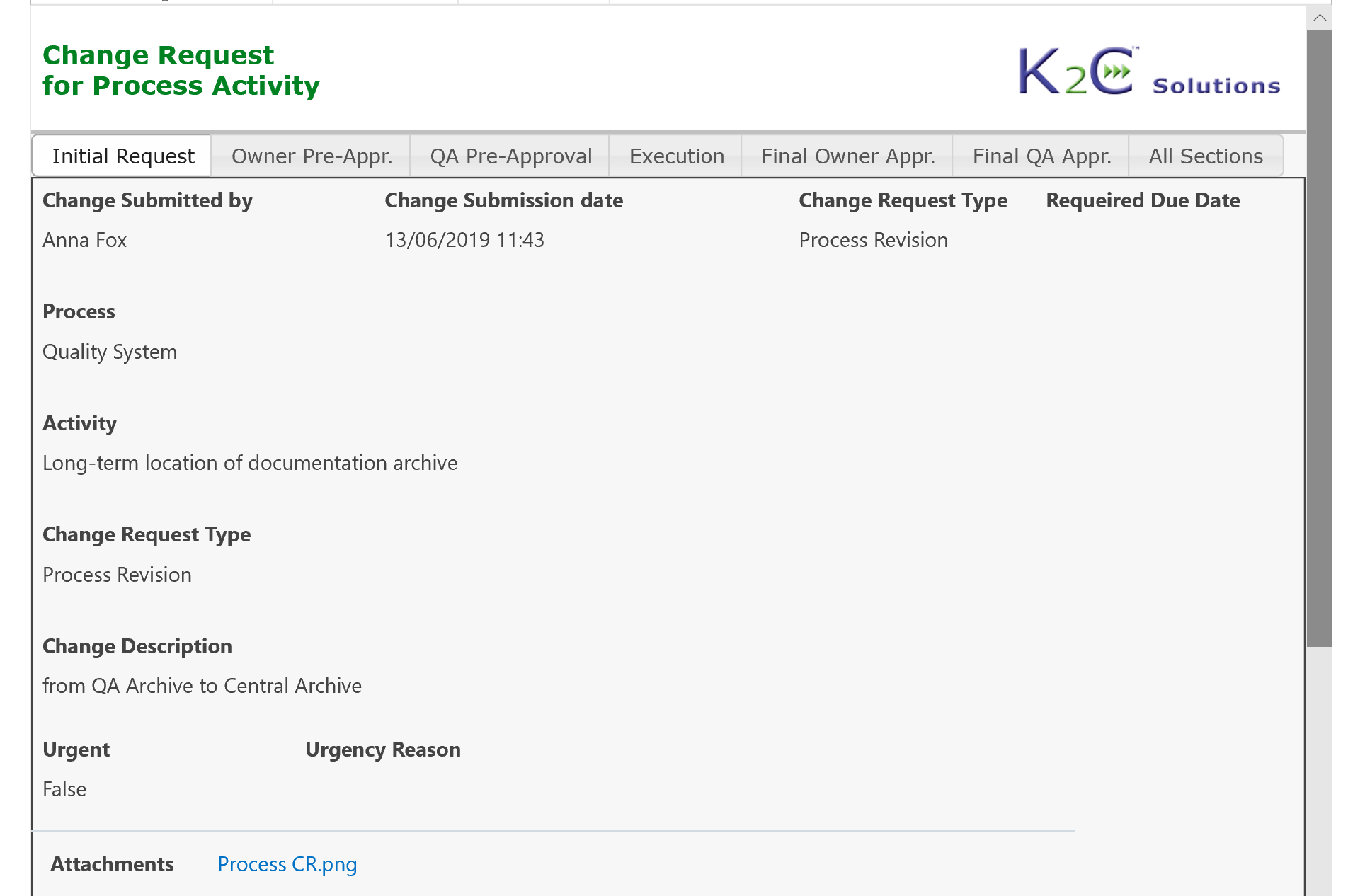
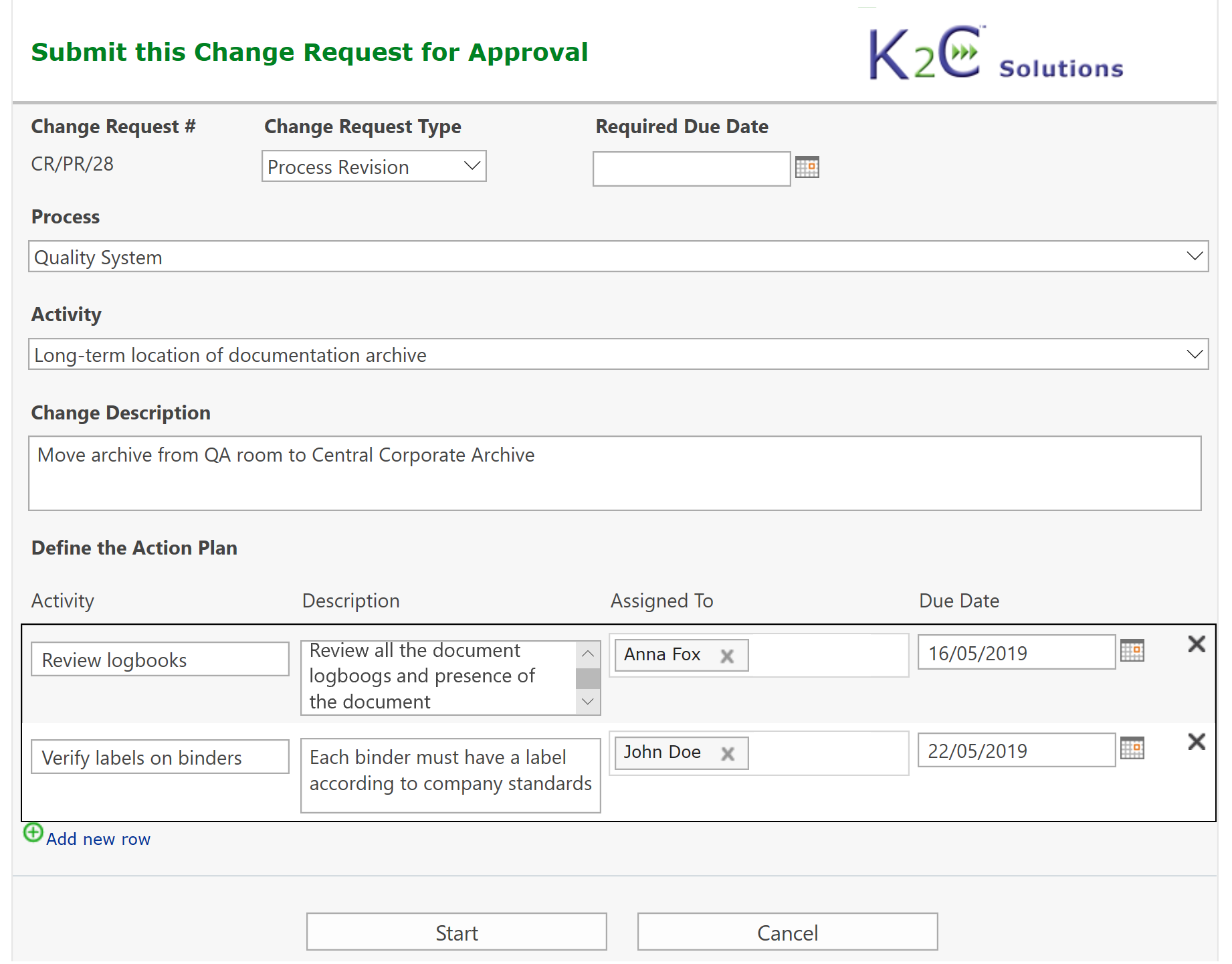
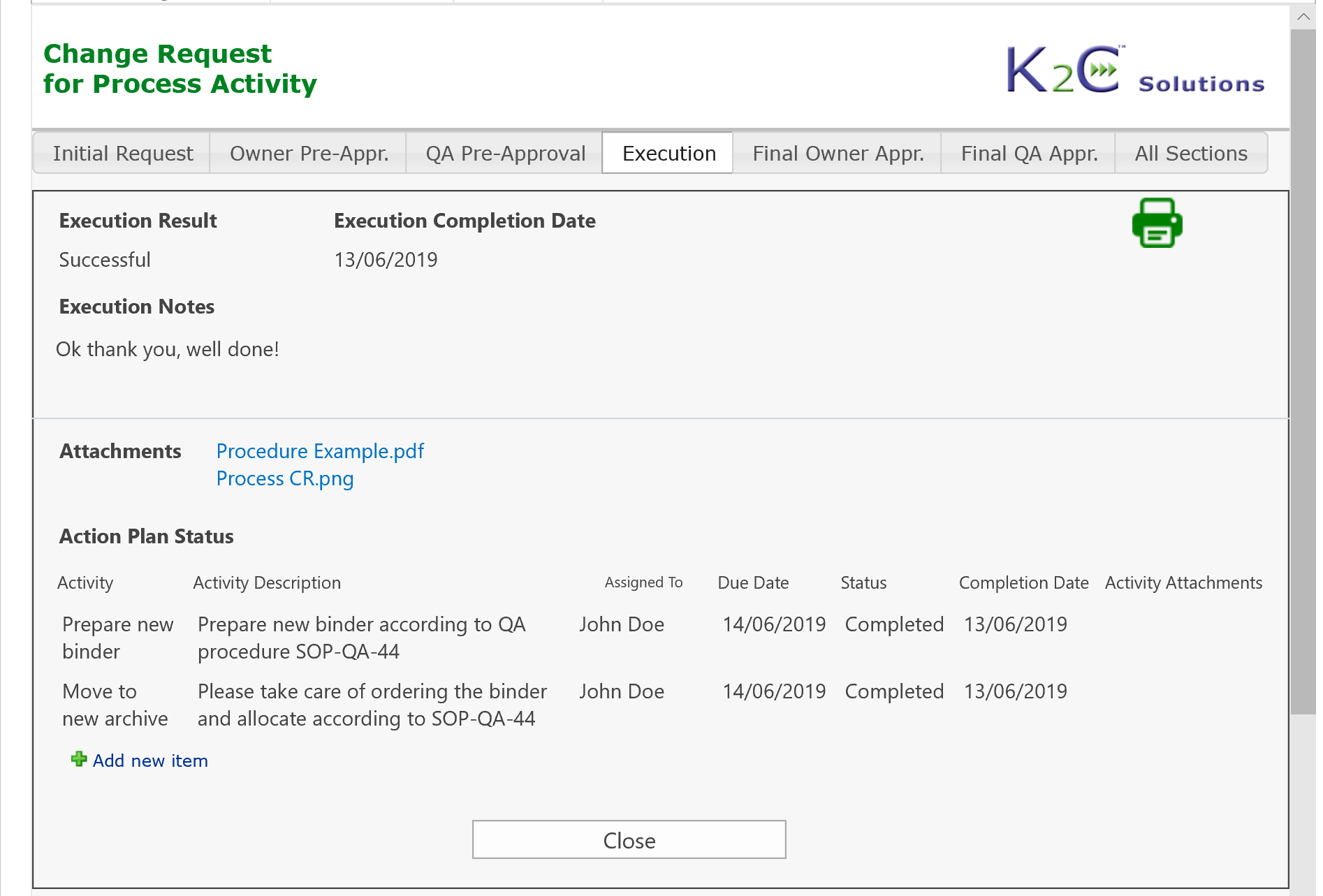
Each system (equipment, utilities, software etc.) can be inventoried, assessed and maintained under control, from the purchasing up to testing, validation. go-live and retirement, tracing at any time its status and speeding-up the more time-consuming activities.
The solution also allows to automate the identification of required documentation for the system, applying pre-defined rules that can be customized as per company standards: this includes both technical and validation documentation, required for those companies, such as Pharmaceuticals manufactures or Life Sciences organizations, that need to comply with security, regulations and other best practices or standards.
Process change requests can be managed hierarchically considering related sub-processes or activities: users can submit a change request for a process and related activities fully automating the routing of the request, that is managed by various steps including planning of required activities, risk assessment, assignment of tasks, monitoring of execution and approvals.
K2C Change Managemnt Solution includes the following main features:
-
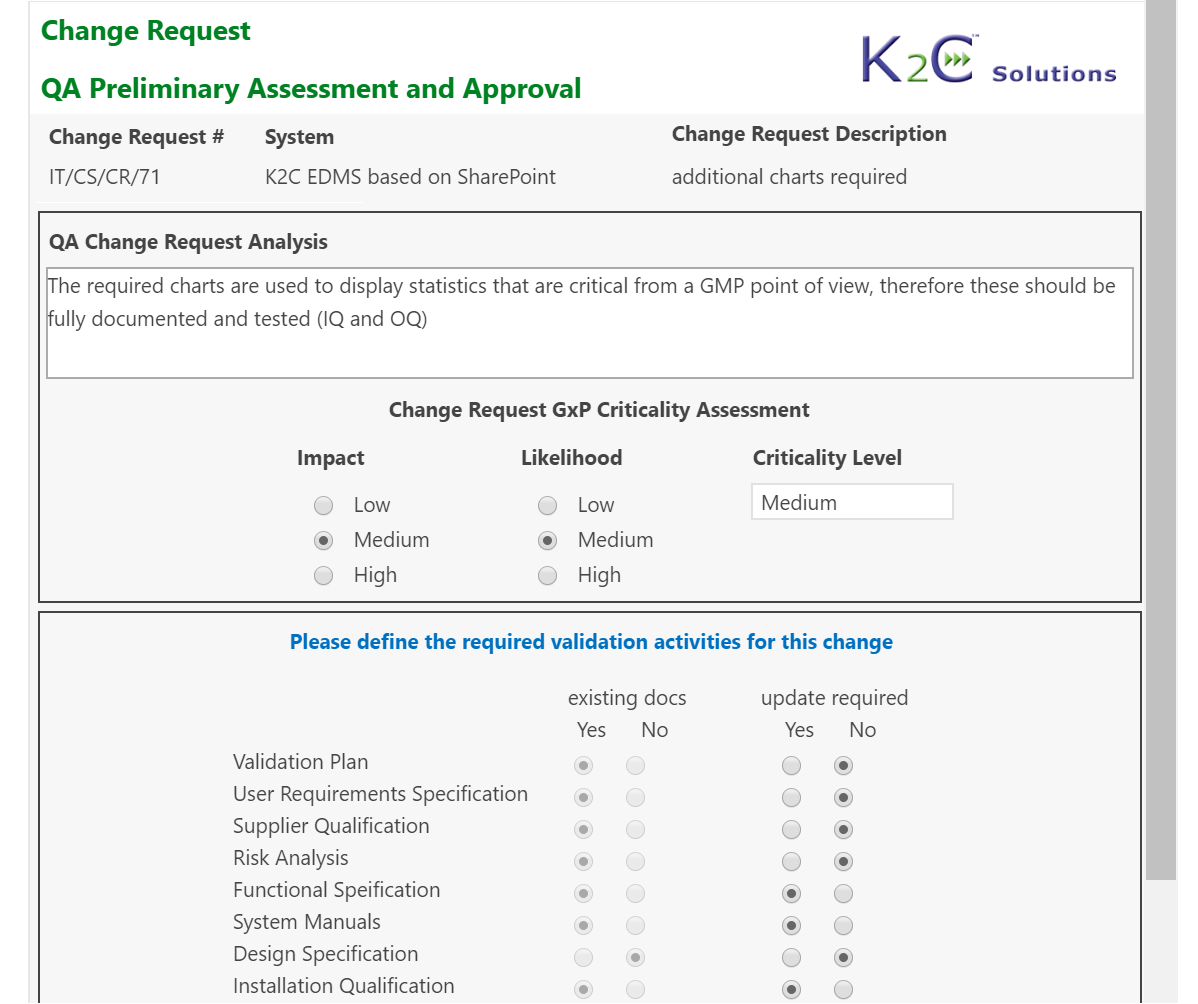
System Assessment: technical classification, documentation and validation requirements, criticality as per applicable standards or regulations
-
Definition of Risk Assessment criteria basing on customizable rules
-
Definition of company policies for required documentation and validation activities
-
Automated definition of documentation and validation requirements, basing on system classification (assessment) and pre-defined company policies
-
Change Request Management, from initial request up to criticality assessment, preliminary approval, planning of required activities, reporting and verification of execution, final approvals and go-live authorization
-
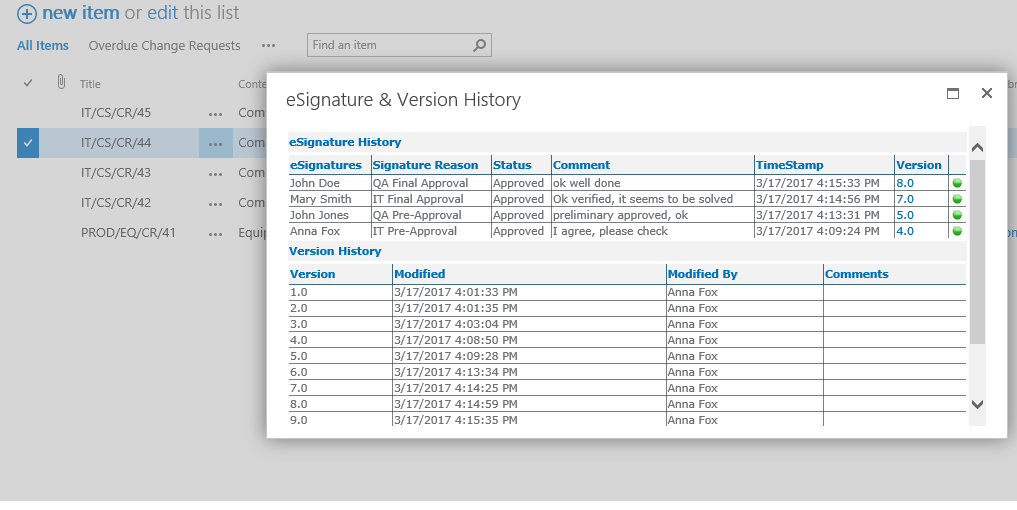
Electronic Signature to ensure a fully paperless management of regulation requirements (21 CFR part 11, Annex 11 etc.) as per the change request of systems used in critical processes
-
Charts, Reports and indicators to monitor in real time the status of each system and related change requests
The solution is available in two alternative versions, respectively based on SharePoint Designer or Nintex workflows.
Contact us for more information or to request for a live demo.